The human body develops most tissue types during fetal development, in a mother’s uterus. Yet only one tissue develops after birth: the mammary gland. This milk-producing organ, a defining characteristic of mammals, is also the site of one of the most common cancers, breast cancer, which affects roughly one in eight women in the United States over the course of their lifetime.
Cancer cells can commandeer molecular pathways used in normal physiological functions to wreak havoc in the body. That is why Rumela Chakrabarti, an assistant professor of biomedical sciences in Penn’s School of Veterinary Medicine, pays close attention to the molecular mechanisms at work in the mammary gland.
“Cancer cells are smart,” she says. “They can actually hijack the normal cellular machinery to use for their benefit. I’m interested in the cellular signaling that is having an impact in normal development as well as in the initiation and development of breast cancer.”
In a paper out this week in the journal Science, Chakrabarti and colleagues report a crucial new signaling exchange that mediates normal mammary gland development by regulating the mammary stem cell niche.
The discovery, the result of years of work that began when Chakrabarti was a postdoctoral researcher at Princeton University in the lab of her co-corresponding author, Yibin Kang, the Warner-Lambert/Parke-Davis Professor of Molecular Biology, indicates that mammary gland stem cells communicate with macrophages, a type of immune cell, using a protein called Delta-like-ligand 1 (Dll1), which is part of the Notch signaling pathway. They find that this molecular chatter is essential for the survival of the mammary stem cells, which leads to mammary gland development. And because the Notch pathway and other molecular components of the communications between mammary stem cells and macrophages have been implicated in breast cancer genesis and spread, future studies of the pathway in the context of cancer may bear crucial information for diagnosis and treatment.
Going into this work, scientists knew that mammary stem cells existed, helping to remodel the breast tissue through the changes associated with puberty, pregnancy, and lactation. But no one had a good way of identifying them, or a solid understanding of their interactions with surrounding cells.
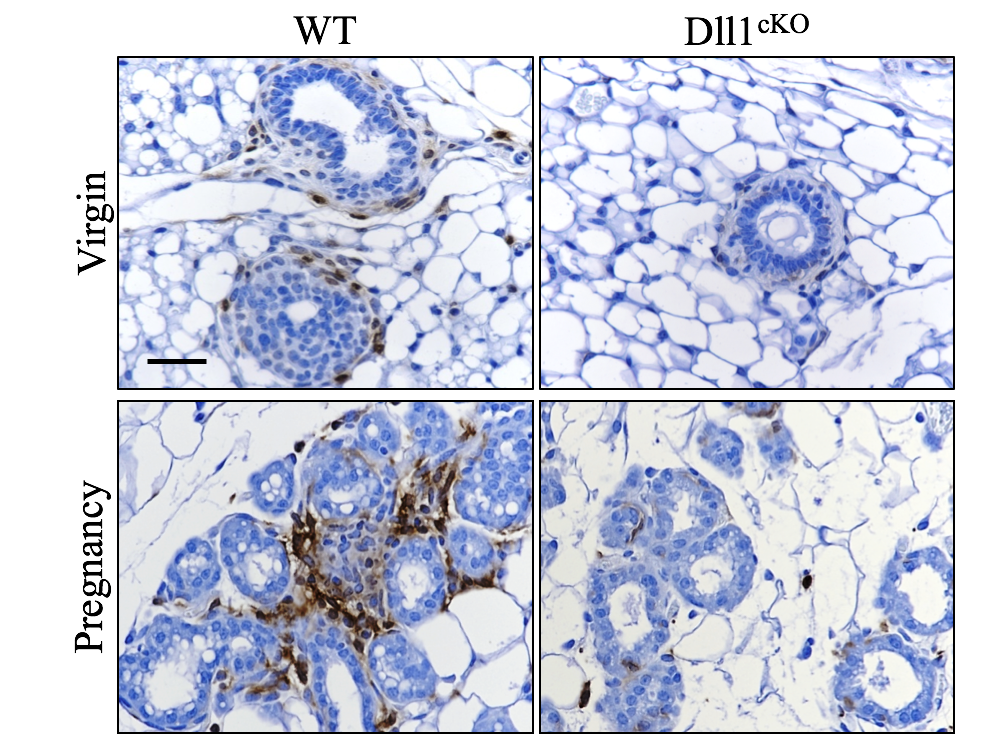
As a first step, Chakrabarti and colleagues compared the gene expression profiles of mammary stem cells versus non-stem cells, and found Dll1 to be among the genes most differentially expressed between the two cell types. Honing in, they developed a mouse model that lacked Dll1 predominantly in the mammary gland. Through every life stage, these mice had problems with mammary gland development, and females did not produce milk after giving birth. They also had significantly fewer mammary stem cells and macrophages compared with normal mice.
“People have identified other genes that affect mammary gland development,” Chakrabarti says, “but usually the defect is temporary and the mammary gland can somehow bypass it over time. With this gene, we found it cannot be compensated, it affects every stage of development, including pregnancy.”
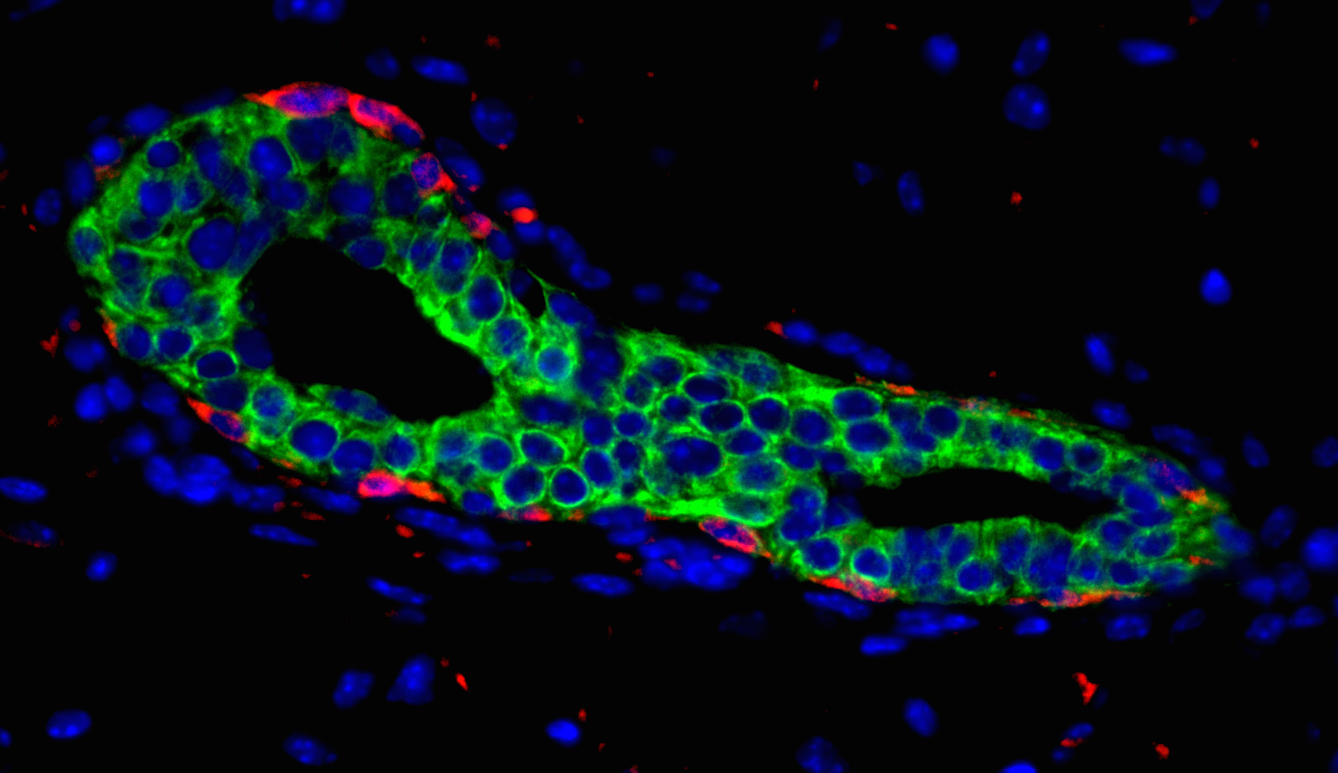
Using additional reporter mouse models, developed by collaborators Hans Clevers of the Netherlands’ Hubrecht Institute and Iannis Aifantis of New York University, the researchers tracked mammary stem cells based on associated florescent color, confirming that Dll1 was indeed a marker of stem cells that were able to give rise to every cell type in the mammary gland.
Because Dll1 was known to be a ligand of Notch signaling, or a molecule that binds to another molecule, the next step was to find its “receiving” molecule. Screening a variety of cell types that exist in the environment of the mammary gland, they narrowed in on macrophages. Working with Ming Li of Memorial Sloan Kettering Cancer Center, who provided mouse models where Notch signaling in macrophages is deleted, they found that mammary stem cells depended on macrophages to function normally.
Further gene expression studies elucidated the relationship, showing that the stem cells used Dll1 to communicate with macrophages, and the macrophages released the signaling proteins, such as Wnt3, 10, and 16 to support the environment around mammary stem cells, allowing them to thrive.
The fact that both Wnt and Notch signaling are involved in supporting the mammary stem cell niche provides a strong clue that the relationship and signaling pathways that link stem cells and macrophages may play a role in breast cancer, as aberrant functioning of both of these pathways have previously been shown to present in breast cancer.
“That is where the lab is now looking,” says Chakrabarti. “How are these pathways functioning in breast cancer?”
If changes in Dll1 expression are found to play a role in the early stages of cancer, Chakrabarti says the molecule offers a promising biomarker and a target for cancer therapy. As a ligand, it could be zeroed in on without the toxicity of some other types of drugs that operate in the same pathway, which function by inhibiting receptors and sometimes have problematic side effects.
“The lab is very interested in detecting early changes in cancer,” Chakrabarti says. “That’s why we are looking very closely at normal development and physiology.” Catching the changeover from normal to malignant at an early stage, she notes, is the best way to save lives.
In addition to Chakrabarti, Kang, Aifantis, Clevers, and Li, the coauthors on the study were: Penn’s Sushil Kumar; Princeton’s Toni Celiá-Terrassa, Xiang Hang, Yong Wei, Abrar Choudhury, Julie Hwang, Jia Peng, John J. Grady, and Christina DeCoste; Memorial Sloan Kettering Cancer Center’s Briana Nixon; New York University’s Jie Gao; and Hubrecht Institute and University Medical Center’s Johan van Es.
The work was supported by the National Cancer Institute (NCI-K22) grant (K22CA193661), Department of Defense (BC103740, BC123187)), the National Institutes of Health (CA193661, CA141062, CA198280, CA008748), the Susan G. Komen Fellowship (PDF15332075), the Brewster Foundation, the Breast Cancer Research Foundation, and the Genomic Editing and Flow Cytometry Shared Resources of the Cancer Institute of New Jersey (P30CA072720).