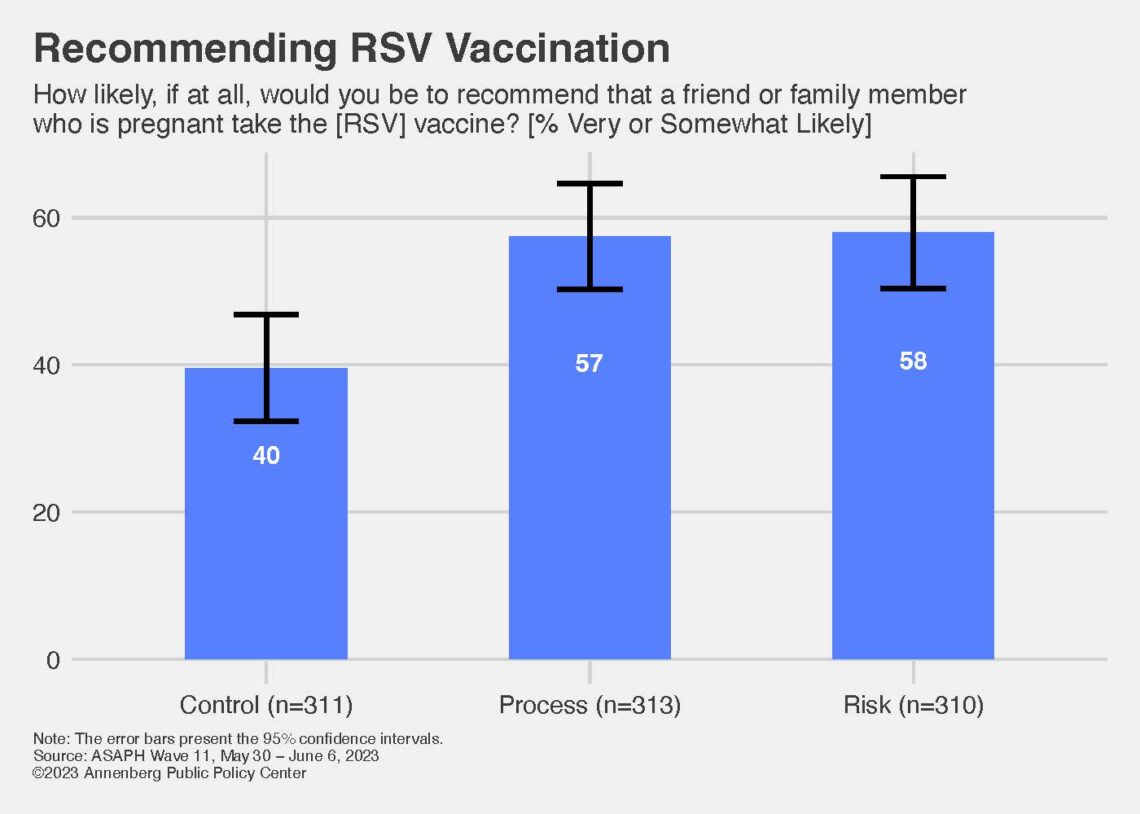
A new study finds that people are more likely to recommend that a pregnant family member or friend get vaccinated to protect the infant from RSV illness if they are shown a chart outlining the rigorous process a vaccine undergoes to be approved by the Food and Drug Administration (FDA).
The experiment was conducted by an Annenberg Public Policy Center (APPC) team as part of a May 31-June 6, 2023, nationally representative panel survey on RSV, vaccination, and maternal health. Researchers found that 57% of those in a group exposed to a flowchart of the FDA vaccine approval process were very or somewhat likely to recommend the RSV vaccine to a pregnant family member or friend, compared with 40% of those in a control group not shown the chart. Those in a third group, informed about the risks of RSV, were also more likely to recommend the vaccine.
RSV or respiratory syncytial virus is the leading cause worldwide of lower respiratory tract infections in babies. Virtually all children get an RSV infection by the age of two. Though typically mild, the highly contagious RSV virus can cause serious illness, hospitalization, and even death among infants and the elderly.
“Over the years the FDA and CDC have developed a sophisticated review system designed to protect the integrity of the data as well as the independence of the analysis on which the vaccination vetting and approval process relies,” says Kathleen Hall Jamieson, director of the Annenberg Public Policy Center and director of the survey. “The public would be well served if the press were to remind the public of this review process when a new vaccine is announced and vigilantly monitor it to ensure that it is doing its intended job well.”
The report, “Reducing susceptibilities to misconceptions about vaccination during pregnancy: RSV,” addresses ways to correct or contextualize misleading or false claims about maternal vaccination in general and vaccination against RSV among those who are pregnant in particular.
The study is part of a new white paper from the Annenberg Public Policy Center that looks at misconceptions about vaccination during pregnancy. “Reducing susceptibilities to misconceptions about vaccination during pregnancy: RSV” is the second in a series of Vaccine Communication and Fact-Checking Toolkit reports produced in partnership with Critica, a nonprofit organization that seeks to improve public understanding and acceptance of scientific evidence and counteract health- and science-related misinformation.
Read more at Annenberg Public Policy Center.