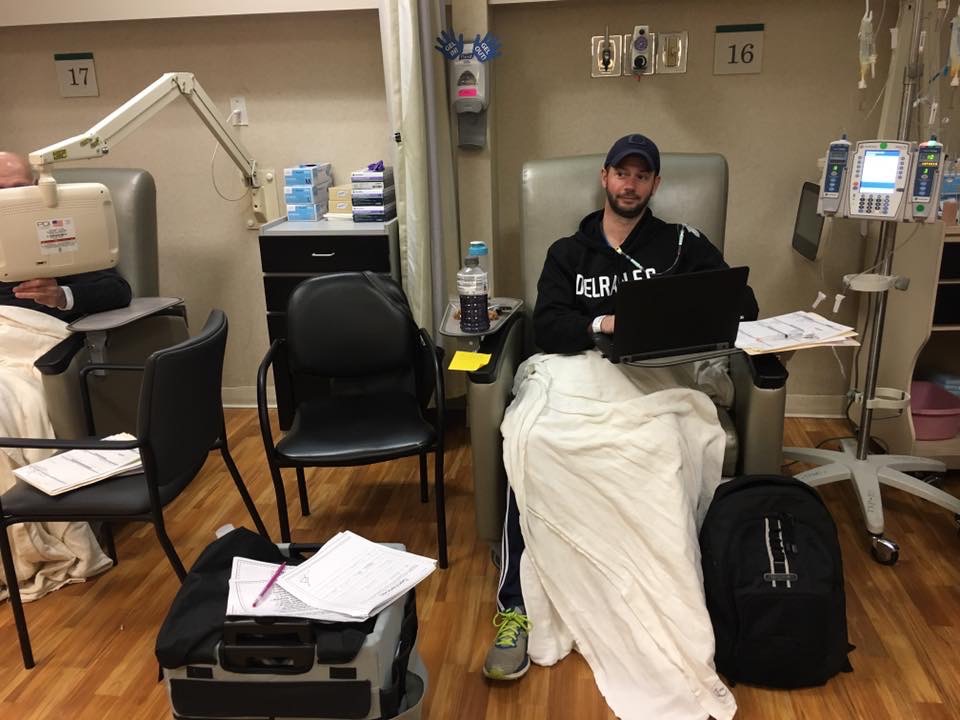
Swept up in a pancreatic cancer diagnosis is inevitably a sense of fear and sadness.
But at Penn, researchers are bringing new hope to this disease. And with patients like Nick Pifani, it’s clear that they’re moving in the right direction.
Pifani, from Delran, New Jersey, first noticed some lingering stomach upset in February 2017. He called his family doctor, concerned—especially given that he was an otherwise healthy marathon runner who was only 42. He was sent to a gastrointestinal specialist. A few weeks later, some crippling stomach pain sent him back to the emergency room and he received an MRI that showed a mass on his pancreas—Stage Three, inoperable, he was told.
He was treated with chemotherapy, along with radiation and, eventually, and after receiving advice from doctors at Penn, his tumor was removed. Thereafter, he realized he had a PALB2 mutation—a cousin of the BRCA gene mutation. At that moment, his long-term needs changed and he found himself seeking specialized care at Penn, where he met Kim Reiss Binder, assistant professor of medicine at the Hospital of the University of Pennsylvania (HUP).
“I’m a planner; I want to understand what [my] potential options are,” Pifani says. “[Reiss Binder] asked why I was there to see her and I explained and quickly I could tell she was—outside of her being remarkably intelligent—a great listener and a compassionate doctor.”
“I have a feeling she worries about me more than I do,” he laughs.
Pifani has now been in remission for two years and four months; he sees Reiss-Binder every three months for checkups. His survival story is inspiring and a sign of momentum, even if a world without pancreatic cancer is still frustratingly out of reach.
Pancreatic cancer at Penn
Pancreatic cancer is the third-leading cause of cancer-related death in the United States, outmatched only by lung cancer (No. 1) and colorectal cancer (No. 2). A person diagnosed with pancreatic cancer is still unlikely to survive past five years—only 9% of survivors do, giving it the highest mortality rate among every major cancer.
In short, pancreatic cancer seldom paves the way for optimistic narratives. Some of the hope that has surfaced, though, is thanks to some talent, dedication to the cause, and hard work at Penn.
A key point of progress in the battle against the disease was made in 2002, when former Assistant Professor of Medicine David Tuveson established a standard model for examining human development of this disease in mice. This model has allowed for a reliable way to study the disease and has influenced progress made here at Penn and elsewhere since.
“There’s been a burst of activity in translational research, from bench to bedside,” explains Ben Stanger, the Hanna Wise Professor in cancer research and director of the Penn Pancreatic Cancer Research Center (PCRC) at the Abramson Cancer Center.
“And there’s a lot of momentum with community building, a dramatic increase in patient volumes, and a dramatic increase in what we know about the cancer,” he says of the status of pancreatic cancer today.
Reiss Binder, meanwhile, explains that one mark of progress at Penn and beyond has been learning about people like Pifani, who have the PALB2 gene, and why they respond differently to treatments than those without it. Platinum-based chemotherapies, for example, are especially effective for people with the PALB2 gene who are battling pancreatic cancer. An ongoing trial at Penn has tested and found some success with using PARP inhibitors—taken orally as an enzyme that fixes single-stranded breaks of DNA—as a maintenance therapy in that same PALB2 demographic after they’ve had chemotherapy. These are less toxic than chemotherapy for patients with the same mutations.
It’s all been slow progress toward better treatments, but there has been progress.
“This is the tip of the iceberg for a disease that we historically have treated with perpetual chemotherapy,” Reiss Binder says. “We owe it to patients to find better options to suppress the cancer but not ruin their quality of life.”
Catching cancer earlier
The consensus on why pancreatic cancer is so deadly? It just can’t be spotted fast enough.
Pancreatic cancer often presents well after it has developed and metastasized, and does so in a way that is not easy to recognize as cancer. Common symptoms include, for example, stomach upset and back pain. And by the time a harder-to-ignore symptom of the cancer surfaces, a sort of yellowing of the skin (a result of a bile duct blockage), it’s likely too late to stop the cancer in its tracks.
One approach to improved detection being tested at Penn, by Research Assistant Professor of Medicine Erica Carpenter, is a liquid biopsy—drawn from a standard blood test. Current means to test for pancreatic cancer—imaging through an endoscopic tube—are invasive and expensive, meaning a common liquid test could transform how many cases are detected early.
Carpenter explains that circulating tumor cells (CTCs) can shed from a tumor that’s adjacent to the wall of a blood vessel; what’s shed then shows up in a blood test. The cells, if detected, can explain more about the nature of the tumor, giving doctors an opportunity to examine characteristics of cancerous cells and decide how to effectively treat a tumor if it can’t be surgically removed. It also allows interpretations of disease burden and the effectiveness of medications—through genome sequencing—that imaging does not.
Ultimately, this gives doctors the potential to track the growth of a tumor before it’s fully developed, all through one tube of blood—detected through an innovative use of technology.
David Issadore, associate professor of bioengineering and electrical and systems engineering in the School of Engineering and Applied Science, has worked since 2017 to develop a chip that detects cancer in the blood, using machine learning to sort through literally hundreds of billions of vesicles and cells, looking for these CTCs. The chip retrieves data and the machine learning developed interprets that data, attempting to make a diagnosis that not only finds pancreatic cancer but also provides information about its progression—and, importantly, whether a patient might benefit from surgery.
Right now, that test has a 24-hour turnaround, he says, but could eventually advance to having a one-hour turnaround. That would be a remarkable mark of progress for discovering the disease earlier when the chip enters a commercial stage.
“Pancreatic cancer is a tough disease, and catching it early is hard,” Issadore acknowledges. “So, we think optimistically but also very cautiously, knowing what a challenging disease it’s been to make progress on, which is what drew us to the disease in the first place.
“I’m not an oncologist,” he adds, “but I’m a bioengineer, and people like us who have a different perspective, the hope is we can do something truly [novel] to shift the [state of the disease].”
He would eventually like to test the chip in people with other types of cancers, like lung, bladder, and liver.
For now, Penn still uses imaging as the standard of care but Carpenter is confident that blood testing is “where we’re heading,” starting with at-risk patients with diabetes and other risk factors.
“The most important thing with this would be that when you put a patient on therapy, it’s good to know as early as possible how likely it is they’re going to respond,” she explains. “Tumor markers are increasingly valuable because you can avoid toxicity of the therapy, the expense of it, and most importantly you then have the opportunity to put the patient on something that might have more of an effect for them.
“The challenge,” she adds, “is in pancreatic cancer we don’t have that many effective therapies.”
Another challenge, she adds, is to find the presence of exosomes, small pieces of tumor cells released into the blood stream, which she says are found in abundance among people with pancreatic cancer and could particularly be targeted among people living with diabetes or an intraductal papillary mucinous neoplasm (IPMN). So, at-risk candidates who may not present with the disease currently but are at risk. Several clinical studies and trials are currently taking place at Penn evaluating this.
A related area of interest is determining if people with diabetes, in particular, are developing cancer as part of the diabetes, or developing diabetes from the cancer. Risk factors—diabetes, genetic markers, etc.—continue to be an important area of study with pancreatic cancer.
Pancreatic cancer and immunotherapy
Immunotherapy is rapidly changing the way patients are treated. And interest in immunotherapy for pancreatic cancer is growing exponentially.
But, it’s complicated.
We are still learning about the immune system in pancreatic cancer, explains Gregory Beatty, assistant professor of medicine and director of the Pancreatic Cancer Clinical Trials Program within the PCRC.
“On one hand, we know that inflammation in the pancreas is a driver of pancreatic cancer. But we also know that T cells in the immune system can attack pancreatic cancer,” he says.
The challenge that has surfaced is that T cells in patients living with pancreatic cancer are often weakened or slowed down; they don’t divide or proliferate very well; and they have a hard time finding the cancer. That makes harnessing them for therapy a challenge. One idea, though, is to engineer one’s own T cells (as in CAR T therapy), while they’re still healthy, to detect and kill pancreatic cancer cells.
Penn recently completed a trial in ovarian cancer, mesothelioma, and pancreatic cancer, using CAR T cells engineered to recognize a protein called mesothelin, which is expressed by pancreatic cancer. The team found that the T cells, when injected into the blood of patients, were safe but had limited activity.
“These CAR T cells can kill pancreatic cancer in the lab really well. But why they don’t do so in patients still remains a mystery,” Beatty says.
It does prove that pancreatic cancer evades the immune system extraordinarily well.
Penn investigators have also done work on CD40, a protein expressed in a wide range of immune cells, explains Robert H. Vonderheide, director of the Abramson Cancer Center, who has been working with Mark O'Hara, an assistant professor of Hematology-Oncology, on CD40 therapeutics. Patients are responding to treatment with CD40—a protein that activates T cells to work more steadfastly and seek out cancer cells.
“It seems to make chemo work better,” Beatty explains.
“This is a very promising treatment for convincing the immune system to attack pancreatic cancer,” Beatty adds, “And in the lab, we are finding ways to make it work even better.”
The larger idea is to build on a backbone of chemo and CD40 in the future to help coax T cells to work better. Overall, a major thrust of treatment for patients at the PCRC is focused on unraveling ways to use immunotherapy while developing the next-generation of strategies for patients with BRCA 1 and 2 genes who are receiving PARP inhibitors.
Navigating a tough battle
The stress of a pancreatic cancer diagnosis can be dizzying. It is, says Pancreatic Nurse Navigator Trish Gambino, a cause to act fast.
“We really believe pancreas cancer is a medical emergency much like a heart attack,” she says. “As a nurse navigator, I try to get newly diagnosed patients with pancreatic cancer expeditiously to the correct provider for staging and treatment.”
Because of that, she says, patients are often still digesting their diagnosis while also juggling appointments, choosing a doctor, making decisions about care, settling personal matters, and communicating with insurance companies. Gambino, one of eight nurse navigators hired to put organization and compassion on the frontlines, takes multiple incoming calls—as many as five—per day from people who have been diagnosed and sound shell-shocked.
“I get so many of these calls per week saying, ‘Trish, I just went to the doctor and they told me I have a pancreatic mass on my CAT scan. And I don’t know what to do,’” she says. “A lot of times patients don’t know what they need.”
Her job is one of compassion but also pragmatism. She listens and places their concerns in context and individualizes her approach to moving patients in the right direction, laying out all the options and giving them a sense of order and control over their narrative.
“It really does take a village to try to get people through this,” Gambino explains, noting how overwhelming the cancer experience can be. “When you have pancreas cancer, it’s not just the medical oncologist, the radiation oncologist, the surgeon, the dietician, the social worker, the nurse navigator, the infusion nurses, the nurse practitioners—they’re all there and the response is often ‘Who is everybody?’ They need someone who can lead the team for them.”
She says that Penn is especially well-regarded for its interdisciplinary teams—even factoring in diet and financial wellness—and their ability to act swiftly. Penn, for instance, performs more than 150 pancreatic cancer surgeries per year and is practiced at it—not typical of every hospital and a draw for newly diagnosed patients who are eligible for resection.
What’s next
Looking ahead, Stanger is optimistic about advances in screening and immunotherapy treatment—particularly research funded by the Parker Institute for Cancer Immunotherapy, started by Sean Parker, a cofounder of Facebook. Penn is one of 10 sites of major investment for research and was the impetus for the investment in pancreatic cancer.
He’s also encouraged that the research community surrounding pancreatic cancer is “collaborative,” he says, with many doctors recognizing the enormous challenge of the disease and working together well.
Celebrity diagnoses, like that of Alex Trebek, als0 lend some hope in the messaging of how the disease is presented to the world today.
“I talk to people almost every day, and when we talk about pancreatic cancer they say, ‘Oh, that’s a really bad one,’” he says. “One thing I respect about Alex is he came out and was very forthcoming and he spoke with a great deal of confidence and hope in the medical community and gave a positive message that said, ‘I’m going to do my best to beat this.’”
Pifani, meanwhile, more than two years out from his surgery, is feeling optimistic. He’s mostly resumed a normal life—with occasional side effects that linger, of course, and scans every six months. He runs marathons and spends time with his wife and kids. And, a member of the Survivor Council at the Pancreatic Cancer Action Network and sponsorship chair for the Philadelphia affiliate, he shows up to community events built around raising awareness of the disease and advocating research and caregiver support.
At Penn, he says, he feels like he’s in the right place with his care—that he’s “in the best hands” if something does happen, and recognizing the disease’s ongoing presence in his life.
“I got a long way to go,” he says, “but we’re off to a good start.”
Homepage photo: Gregory Beatty, assistant professor of medicine and director of the Pancreatic Cancer Clinical Trials Program within the Penn Pancreatic Cancer Research Center, examines a blood sample.