
Image: Aditya Irawan/NurPhoto via AP Images
Working with yeast and worms, researchers found that incorrect gene expression is a hallmark of aged cells and that reducing such “noise” extends lifespan in these organisms. The team published their findings this month in Genes & Development.
The team was led by senior author Shelley Berger, PhD, a Daniel S. Och University Professor in the departments of Cell & Developmental Biology, Biology & Genetics at the Perelman School of Medicine at the University of Pennsylvania, and Weiwei Dang, PhD, a former Penn postdoctoral fellow who is now an assistant professor at Baylor College of Medicine, along with first author Payel Sen, PhD, currently a postdoctoral fellow in the Berger lab. Berger is also director of the Penn Epigenetics Program.
Gene expression is regulated by chemical modifications on chromatin -- histone proteins tightly associated with DNA. Certain chemical groups on histones allow DNA to open up, and others to tighten it. These groups alter how compact DNA is in certain regions of the genome, which in turn, affect which genes are available to be made into RNA (a process called transcription) and eventually proteins.
“Researchers have just started to appreciate how these epigenetic histone modifications may be playing essential roles in determining lifespan,” said Berger. She has been studying such epigenetic markings for over two decades and was among the first to pinpoint specific histone modifications that not only are altered during aging, but also directly determine longevity.
“In this study, we found that a type of abnormal transcription dramatically increases in aged cells and that its reduction can prolong lifespan,” said Dang, who initiated this line of research while working in Berger’s laboratory. “This longevity effect is mediated through an evolutionarily conserved chemical modification on histones. This is the first demonstration that such a mechanism exists to regulate aging.”
Click here to view the full release.
Karen Kreeger

Image: Aditya Irawan/NurPhoto via AP Images

nocred
Image: Michael Levine
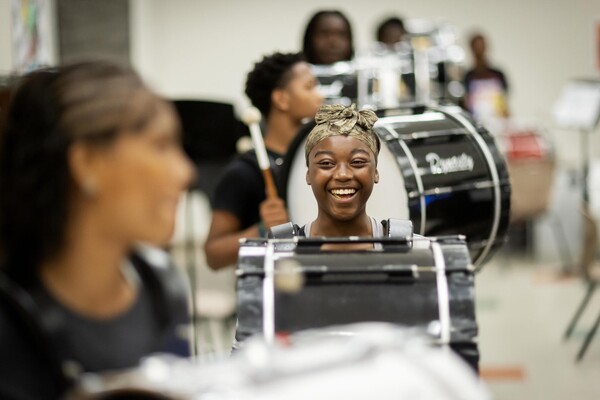
A West Philadelphia High School student practices the drum as part of a July summer program in partnership with the Netter Center for Community Partnerships and nonprofit Musicopia.
nocred