
Image: Aditya Irawan/NurPhoto via AP Images
PHILADELPHIA — An experimental vaccine developed by researchers at the University of Pennsylvania’s schools of Medicine and Veterinary Medicine is the first veterinary cancer vaccine of its kind that shows an increase in survival time for dogs with spontaneous non-Hodgkin’s lymphoma. The work shows for the first time the feasibility and therapeutic efficacy of this alternative cell-based vaccine, which could be employed in the treatment of a number of different cancer types.
The research was conducted by Nicola Mason, assistant professor of medicine at Penn Vet; Robert H. Vonderheide, associate professor of hematology and oncology at the Perelman School of Medicine; and Karin U. Sorenmo, associate professor of oncology at Penn Vet. Erika Krick, Beth Overley and Thomas P. Gregor of Penn Vet and Christina M. Coughlin of the School of Medicine also contributed to the research.
Their work was published in the open access journal PLOS ONE.
The team recruited dogs that were brought to Penn’s Matthew J. Ryan Veterinary Hospital with newly diagnosed non-Hodgkin’s lymphoma to receive the experimental vaccine following standard induction chemotherapy and confirmation of clinical remission. The goal of the study was to determine whether the vaccine would prevent or prolong time to a relapse, a common scenario in both humans and dogs with NHL.
“We vaccinated dogs, which were in clinical remission following chemotherapy, three times,” Mason said. “We then tracked them over several years to see if the vaccine would prevent relapse and would prolong overall survival.
“We found that, although the vaccinated dogs still relapsed with clinical disease when they were treated with rescue chemotherapy, they had significantly increased overall survival times when compared to an unvaccinated control group. Some of these dogs are still alive and cancer free more than three years later.
“The results with these dogs indicate that our immunotherapy and rescue chemotherapy appear to act synergistically to prevent a second relapse – a phenomenon that has been previously recognized in human patients treated with other types of immunotherapy,” she said.
Previous cell based vaccines have utilized genetically engineering dendritic cells — which are part of the immune system — to stimulate immune responses against cancers. Similar to using weakened viruses in traditional vaccines, scientists load these cells with tumor proteins and inject the cells back into the patient’s body. Such cell-based vaccines are already being used to treat prostate cancer in humans, but engineering these cells is expensive and time consuming. Furthermore, patients must also endure long, leukapheresis sessions in which the necessary dendritic cells are harvested from their blood.
The Penn team hypothesized that another kind of immune cell, B-cells, could work just as well under the right conditions. Unlike dendritic cells, many B-cells can be grown from a small blood sample, removing the requirement for leukapheresis.
Mason’s team made the vaccine by culturing B-cells from the blood taken from the dogs with NHL. These cells were then loaded with RNA that had been isolated from the patient’s own tumor.
The results were impressive.
“Though vaccinated and unvaccinated dogs relapsed with clinical disease at the same time, 40 percent of vaccinated dogs that relapsed experienced long-term survival after a second round of chemotherapy; only 7 percent of unvaccinated dogs that relapsed and were treated with the same rescue chemotherapy protocol survived long term,” Mason said. “Furthermore, when the vaccinated long-term survivors did eventually die, they showed no evidence of lymphoma on full necropsy.”
While the molecular mechanisms responsible for these observed synergistic effects are currently unknown, Mason believes that the vaccine-primed immune system may be boosted by the effects of rescue chemotherapy leading to long term second remissions.
Though the increases in long-term survival are already unprecedented and the proof-of-concept for B-cell-based cancer vaccines represents a step forward in cell-based vaccine development, future research could have even more exciting results.
“These dogs just received three doses of vaccine, three weeks apart. If we kept boosting the immune system in this way by vaccination, perhaps the dogs would not relapse in the first place,” Mason said.
Work is now underway to streamline B-cell vaccine generation and initiate further clinical trials aimed at optimizing this novel cell-based approach.
This work was supported by the National Institutes of Health, Alliance for Cancer Gene Therapy, Onyx and Breezy Foundation, Barry and Savannah Poodle Memorial Fund, Mari Lowe Comparative Oncology Center, Immunobiology Program of the Abramson Cancer Center at the University of Pennsylvania and Oncology Research Fund at the Veterinary Hospital of the University of Pennsylvania.
Evan Lerner

Image: Aditya Irawan/NurPhoto via AP Images

nocred
Image: Michael Levine
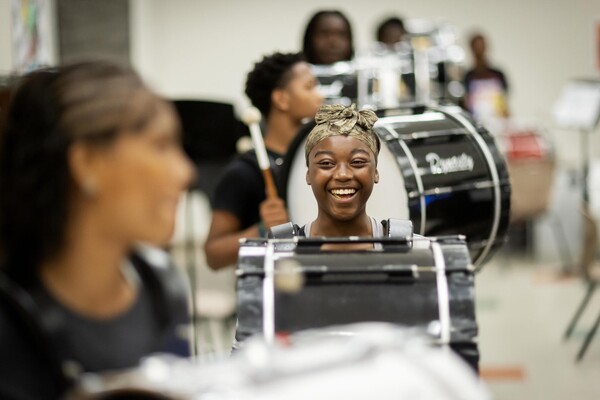
A West Philadelphia High School student practices the drum as part of a July summer program in partnership with the Netter Center for Community Partnerships and nonprofit Musicopia.
nocred