
Image: Aditya Irawan/NurPhoto via AP Images
A second study of the injectable anti-obesity medication, semaglutide, has confirmed the large weight losses reported in a study earlier this month, establishing the reliability and robustness of this new drug. With obesity affecting more than 40% of American adults, the findings could have a major impact on weight management in primary care and other settings. The study is published in the Journal of the American Medical Association.
The 68-week study was conducted at 41 sites in the United States from August 2018 to April 2020 and was designed to boost total weight loss with semaglutide by combining the medication with a more intensive diet and physical activity program than what was used in the STEP 1 trial. All participants in the new STEP 3 study received 30 sessions of intensive behavioral therapy consisting of diet and physical activity counseling, which was combined with an initial 8-week, 1,000-1,200 kcal/day meal-replacement diet, consisting of shakes, meal bars, and prepared entrees.
“We wanted to induce a large weight loss with rigorous behavioral therapy and see how much additional weight loss semaglutide could add,” says lead author Thomas Wadden, a professor of psychology in the at the Perelman School of Medicine.
This story is by Sophie Kluthe. Read more at Penn Medicine News.
From Penn Medicine News

Image: Aditya Irawan/NurPhoto via AP Images

nocred
Image: Michael Levine
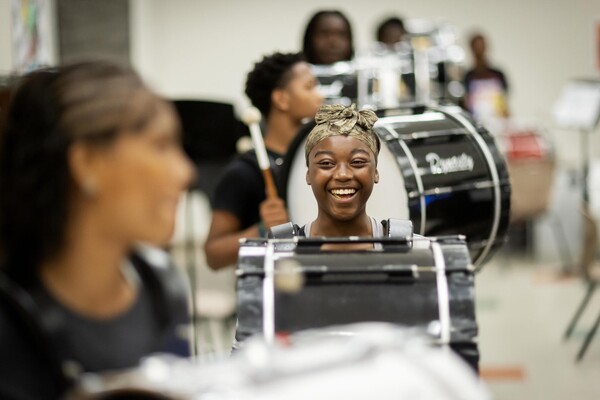
A West Philadelphia High School student practices the drum as part of a July summer program in partnership with the Netter Center for Community Partnerships and nonprofit Musicopia.
nocred