
(From left) Doctoral student Hannah Yamagata, research assistant professor Kushol Gupta, and postdoctoral fellow Marshall Padilla holding 3D-printed models of nanoparticles.
(Image: Bella Ciervo)
For cancer to spread, it needs a hospitable environment in distant organs. This fertile “soil” can provide a home to circulating malignant cells. Recent research has shown that cancer cells from the primary tumor can help ready this soil by sending out small vesicles. These vesicles contain a cocktail of molecules that “educate” healthy cells to prepare the target tissues for cancer cells to seed and thrive. Blocking this process offers one strategy to stop metastasis, which is often responsible for cancer’s lethality.
New research from the University of Pennsylvania’s School of Veterinary Medicine has identified an FDA-approved drug that, when used with surgery, hampers metastasis in an animal model. Originally developed and approved nearly 65 years ago to control blood pressure, the medication reserpine also prevents what are known as tumor-derived extracellular vesicles (TEVs) from fusing to healthy cells and sharing their cargo of disease-promoting molecules, the research team found.
“No matter what we do to kill cancer cells—surgery or radiotherapy or chemotherapy—causes stress, and the data show that the stress can stimulate production of these vesicles,” says Serge Fuchs, a biologist at Penn Vet. “So our thinking is, as an adjuvant to that primary treatment, it would be prudent to limit the effect of these vesicles on healthy tissues, thereby preventing the spread of malignant cells.”
The study’s findings, published in the journal Cancer Cell, show that giving moderate doses of reserpine to mice with melanoma prior to and following surgery disrupted the uptake of TEVs by healthy cells, reduced the spread of the cancer, and significantly prolonged survival in the animals.
While previous research had shown that TEVs encourage metastatic disease and, in some circumstances, can transform normal cells into malignant ones, it’s clear that not every healthy cell that comes into contact with these vesicles becomes cancerous. Thus scientists have hypothesized that healthy cells may possess a strategy to defend themselves against this transformation. Such a defense mechanism could be a target for anti-metastatic therapies.
In search of the players in a putative defense, Fuchs and colleagues looked to see which surface proteins on human cells changed in number upon exposure to TEVs from melanoma cells. One of the more significant to undergo a change was IFNAR1, one of the proteins that compose the type I interferon receptor, a receptor which Fuchs has studied previously. Interferon is used as a cancer therapy, including against malignant melanoma, and is known to play a role in preventing cancer’s spread.
In lab experiments using human cells in a dish, treatment with vesicles from melanoma patients, but not from healthy people, decreased IFNAR1 levels. Looking at clinical data, the researchers found that human melanoma patients with metastatic disease were more likely to have lower levels of IFNAR1.
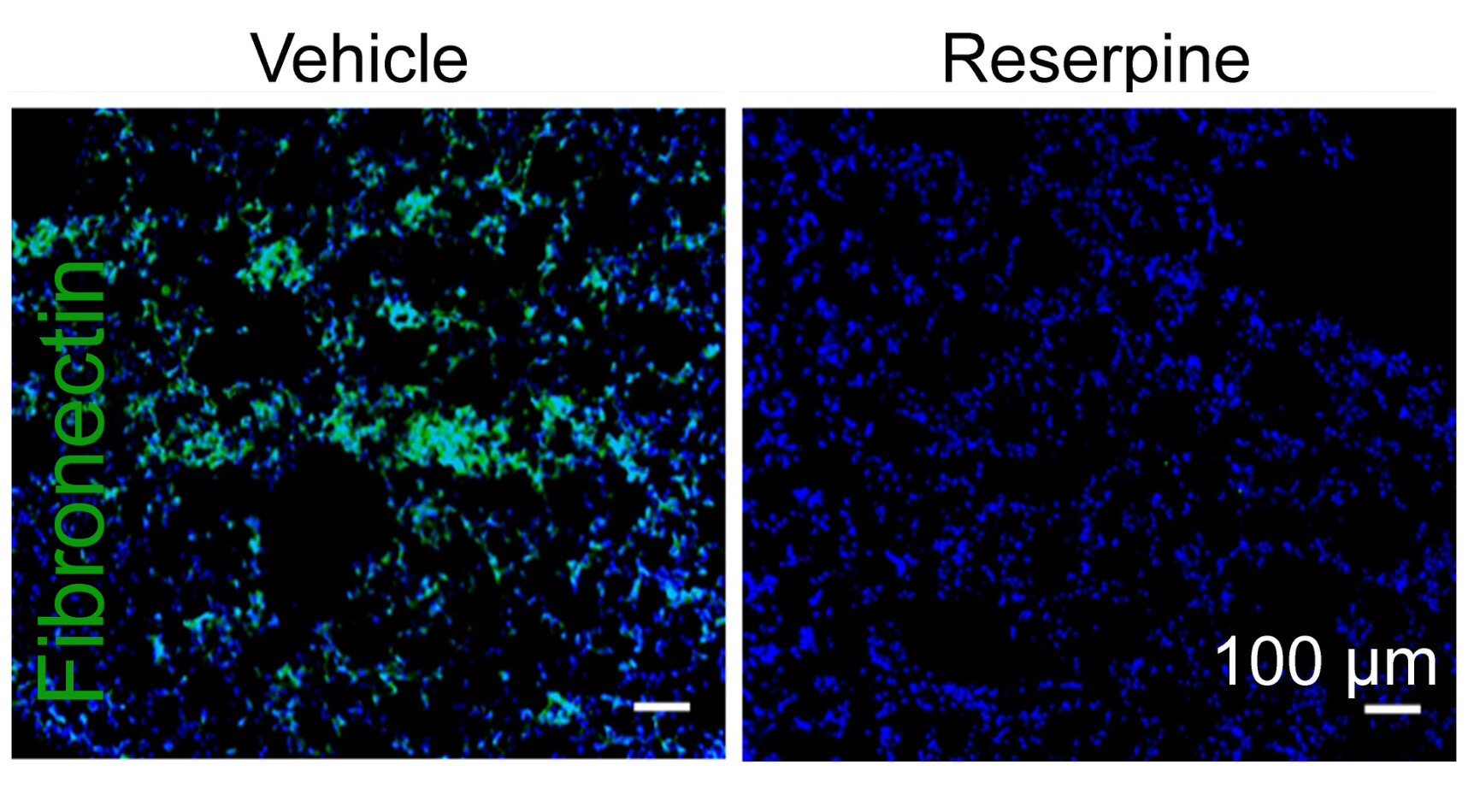
To understand how TEVs influenced the reprogramming of healthy cells to contribute to a metastatic soil, they used a mouse model possessing a form of IFNAR1 resistant to degradation. These mice, they found, resisted uptake of TEV, and did not develop lung metastases from melanoma tumors. From a series of fortuitous experimental clues, the team discovered that healthy cells from these mice were less likely to take up the TEVs because the lipid membrane of the vesicle did not efficiently fuse with the lipid membrane of the cell.
“At that point, we started thinking that this continuous swallowing of vesicles by healthy cells is important for the ‘education’ of normal cells,” Fuchs says. “That suggested to us that any event that would interrupt this continuous uptake of the vesicles by normal cells might be able to disrupt their reprogramming, and might be antimetastatic.”
The researchers found success in pretreating cells with 25-hydroxycholesterol (25HC), a compound that is induced by interferon and has been shown to disrupt fusion of lipid membranes. Cells exposed to 25HC took up fewer TEVs. Further experiments suggested that the pathway encompassing 25HC and interferon played a role in preventing the TEVs from reprogramming normal cells.
Because 25HC degrades quickly in the body, making it a poor choice for a cancer drug, the scientists continued their search, looking for compounds already FDA approved for use in humans that would similarly disrupt the uptake of TEVs. Their screening turned up reserpine, which had fallen out of favor for the treatment of hypertension after better drugs came onto the market in the decades since its approval.
“Reserpine causes sleepiness and sometimes depression,” says Fuchs. “These side effects are a real nuisance if you’re using it daily as a blood pressure medication, but if I had metastatic melanoma I would certainly be willing to tolerate those effects around the time of surgery.”
Testing reserpine in mice with a melanoma tumor, the researchers administered the drug before and after surgery to remove the primary tumor. While reserpine given alone appeared to have little effect on tumor growth and survival, mice that received the reserpine treatment before and after surgery seemed to disrupt the reprogramming of healthy cells. Overall survival of these animals significant improved, and the treatment “virtually eliminated” evidence of lung metastases, the researchers report.
“We are eager to get this into the hands of medical and veterinary oncologists,” Fuchs says.
To follow up on the promising findings, Fuchs and colleagues hope to explore more in depth the effect of reserpine not only on metastasis, but also on vesicle formation and composition. They’d also like to further explore the role of the enzyme that produces 25HC in health and disease.
Fuchs’s coauthors on the piece were Penn Vet’s Angelica Ortiz, Jun Gui, Pengfei Yu, Christina Cho, Sabyasachi Bhattacharya, Christopher J. Carbone, Qiujing Yu, Kanstantin V. Katlinski, Yuliya V. Katlinskaya, Simran Handa, Victor Haas, Susan W. Volk, and Angela K. Brice; Wei Guo from the Department of Biology in Penn’s School of Arts and Sciences; Constantinos Koumensis of Penn’s Perelman School of Medicine; Kim Wals, Nicholas J. Matheson, Robin Antrobus, and Paul J. Lehner of the Cambridge Institute for Medical Research; Sonja Ludwig, Theresa L. Whiteside, Cindy Sander, Ahmad A. Tarhini, and John M. Kirkwood of the University of Pittsburgh School of Medicine; Hallgeir Rui of the Medical College of Wisconsin; and J. Alan Diehl of the Medical University of South Carolina.
The work was supported by the National Institutes of Health (grants CA165997, CA092900, CA216936, and CA188575), The Commonwealth of Pennsylvania Health Research Formula Funds, The Wellcome Trust, the Medical Reserves Corps, NHS Blood and Transplant, the Pittsburgh-Essen-Partnership Program, the University of Pennsylvania, and the Merial Veterinary Scholars Program Fellowships.
Serge Fuchs is a professor of cell biology in the School of Veterinary Medicine at the University of Pennsylvania.
Katherine Unger Baillie

(From left) Doctoral student Hannah Yamagata, research assistant professor Kushol Gupta, and postdoctoral fellow Marshall Padilla holding 3D-printed models of nanoparticles.
(Image: Bella Ciervo)

Jin Liu, Penn’s newest economics faculty member, specializes in international trade.
nocred

nocred

nocred