
Researchers, including Rahul Singh (left), in the Daniell lab’s greenhouse where the production of clinical grade transgenic lettuce occurs.
(Image: Henry Daniell)
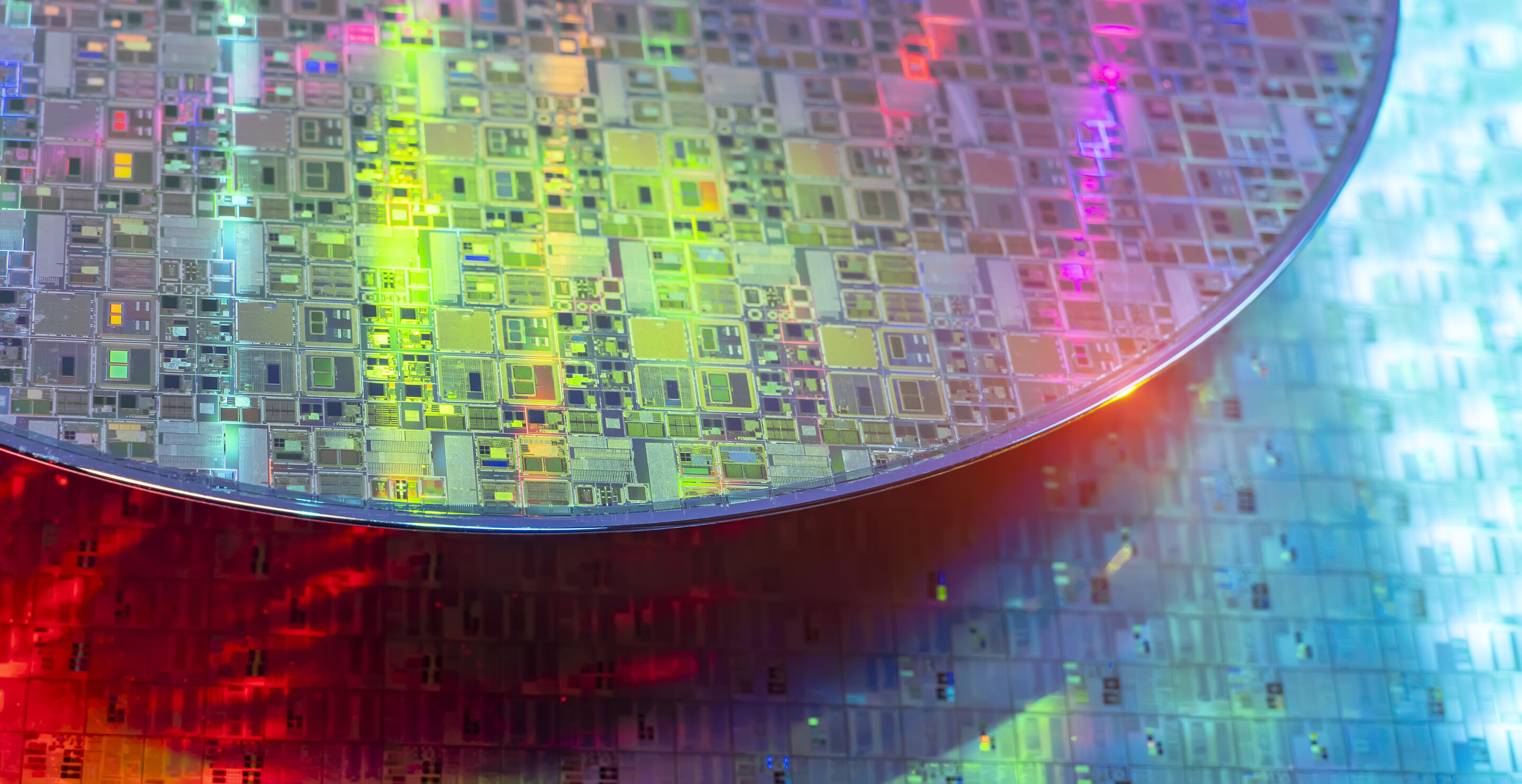
Unlike in humans, when computer “brains” evolve, they get smaller and smaller. This is because the components that perform calculations and consolidate stored information work more efficiently when there are more of them tightly packed on a chip.
Yet when the chip feature sizes get too small, say, to the nanometer scale, their physical and material properties can change, rendering them less reliable at doing their jobs. In the last decade, scientists have made great strides in uncovering new substances that instead become increasingly stable as they scale down, hinting at the promise of smaller storage devices that can be integrated onto silicon computer processing units (CPUs) to increase speed and functionality.
One such compound is hafnium dioxide (HfO2), a material that was found to retain a desirable property, known as ferroelectricity, even at the few-nanometer scale (~2nm). When a ferroelectric material is exposed to a strong enough external electric field, it becomes strongly electrically polarized, which is a state where the material has plus-minus charge dipoles in alignment. What’s great about ferroelectric materials is that this polarization persists, even if the external electric field is removed, analogous to how an iron nail can become permanently magnetized. This persistent polarization means that the material remembers the last direction it was electrically polarized.
What makes HfO2 special is that it can rapidly switch between an up or down mode—corresponding to the ones and zeroes computers use—at reduced dimensions and then retain this information until it is switched again. But how it’s able to achieve this feat has remained a mystery.
Now, a group of researchers led by Andrew M. Rappe, the Blanchard Professor of Chemistry in the School of Arts & Sciences, has uncovered how HfO2 retains its ferroelectric phase in these conditions and explains how it remains stable.
Their research, published in Science Advances, details how HfO2 undergoes a two-step transition resulting in a change in the arrangement of its atoms when grown on a thin film. This allows it to “transition from one phase, which isn’t very useful, to a special one that could be useful for the next generation of information storage devices,” says co-first author of the paper, Songsong Zhou, a postdoctoral researcher in the School of Arts & Sciences.
“The popular belief explaining the mechanism of this phase transition was that it was either a simple single proper phase transition, or a rare and complicated improper phase transition,” says Zhou. “However, we were able to present a third alternative: Tension from being grown on a thin film and an unconventional change in HfO2’s polarization state are linked together to drive a wholly new reaction that induces an antiferroelectric state that actually stabilizes HfO2’s ferroelectric state.”
The ability to have a material be both ferroelectric and antiferroelectric was a major surprise finding. The researchers were under the impression that these were competing states because antiferroelectric materials have their charges alternate between up and down, as opposed to the unidirectional ferroelectric charges. “Our model presents a new framework for understanding phase transitions for materials capable of retaining polarization states at the nanometer scale,” says co-first author of the paper, Jiahao Zhang, a sixth-year Ph.D. student in the chemistry department.
“HfO2 and a few other materials are competing to become successful computer memory materials, but all of them currently have problems,” says Rappe. “In offering a greater insight into the mechanism of ferroelectricity in HfO2, our work addresses some of these issues and paves the way for developing the next generation of materials that could someday soon integrate both processing and memory onto a single chip.”
Next, the researchers will build on their models as they continuously merge experimental and theoretical insights to harness the nanomaterials world.
The research is supported as part of the center for 3D Ferroelectric Microelectronics, an Energy Frontier Research Center funded by the U.S Department of Energy, Office of Science, Basic Energy Sciences (Award DE-SC0021118).
Andrew M. Rappe is the Blanchard Professor of Chemistry in the Department of Chemistry in the School of Arts & Sciences, with a secondary appointment in the Department of Materials Science and Engineering in the School of Engineering and Applied Science. He also co-directs the Vagelos Integrated Program in Energy Research at the University of Pennsylvania.
Jiahao Zhang is a sixth year Ph.D. candidate in the Department of Chemistry in Penn’sSchool of Arts & Sciences.
Songsong Zhou, is a postdoctoral researcher in the Department of Chemistry in Penn’sSchool of Arts & Sciences.

Researchers, including Rahul Singh (left), in the Daniell lab’s greenhouse where the production of clinical grade transgenic lettuce occurs.
(Image: Henry Daniell)

Image: Sciepro/Science Photo Library via Getty Images

In honor of Valentine's Day, and as a way of fostering community in her Shakespeare in Love course, Becky Friedman took her students to the University Club for lunch one class period. They talked about the movie "Shakespeare in Love," as part of a broader conversation on how Shakespeare's works are adapted.
nocred

nocred